Ammonium sulfate Stock Solution
This guide describes the preparation of Ammonium sulfate Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Ammonium Sulfate ((NH₄)₂SO₄) Solution
Ammonium sulfate ((NH₄)₂SO₄) is an inorganic salt widely used in biochemical and analytical laboratories. It dissolves readily in water, forming clear solutions frequently employed for salting‑out proteins, preparing ionic strength adjustment solutions, or general reagent stock solutions. Its solubility in water at 20 °C is high (≈76 g per 100 mL), and the solid appears as white crystalline powder.
Objective
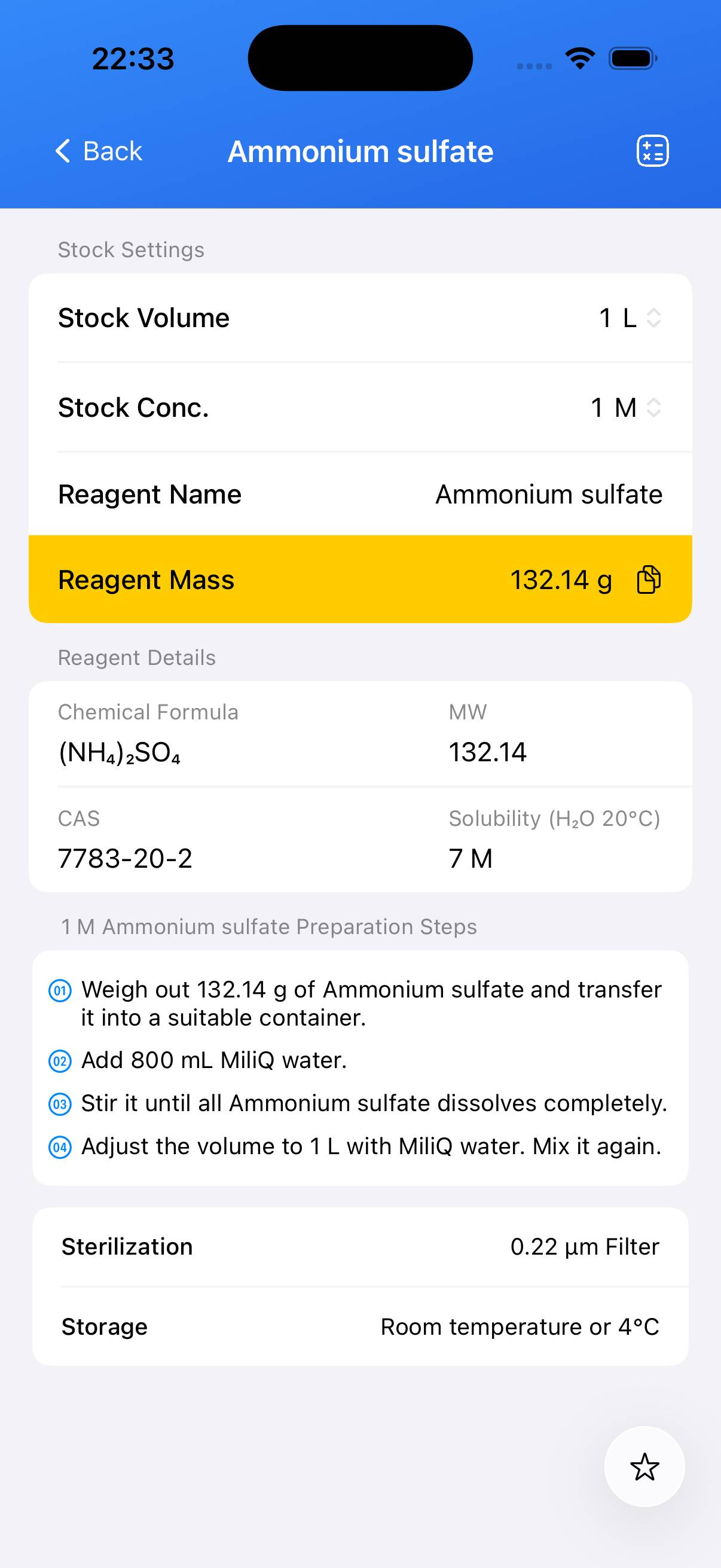
Preparation of a defined concentration stock solution of ammonium sulfate (e.g., 1 M).
Composition
- Ammonium sulfate ((NH₄)₂SO₄) aqueous solution of desired molarity
Required Materials
- Ammonium sulfate ((NH₄)₂SO₄) solid
- Deionized or distilled water
- Analytical balance
- Volumetric flask or graduated cylinder
- Stirring bar or glass rod
- Personal protective equipment (gloves, goggles, lab coat)
Procedure
- Step 1
Decide the target concentration (e.g., 1 M). For a 1 M solution: dissolve **132.14 g** of ammonium sulfate in water and dilute to **1 L** total volume. Adjust the mass proportionally for other final volumes (e.g., 13.214 g for 100 mL of 1 M solution). - Step 2
Add the weighed ammonium sulfate solid to a beaker containing ~80 % of the final desired volume of distilled water. Stir until fully dissolved. - Step 3
Transfer the solution to a volumetric flask and add water up to the final volume. Mix thoroughly. - Step 4 (Optional)
If the solution is intended for sterile applications, filter the solution through a **0.22 µm** membrane into a sterile container.
Note: NH₄⁺ and SO₄²⁻ species contribute to ionic strength; adjust pH only if required by downstream applications. Room temperature storage is generally acceptable for concentrated ammonium sulfate solutions, but clarify any specific requirements for your protocol.
Available Reagents
| Name | Molecular Formula | MW | CAS |
|---|---|---|---|
| Ammonium sulfate | (NH₄)₂SO₄ | 132.14 | 7783‑20‑2 |