Magnesium sulfate Stock Solution
This guide describes the preparation of Magnesium sulfate Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Magnesium Sulfate (MgSO₄) Solution
Magnesium sulfate (MgSO₄) is highly soluble in water. Laboratory stock solutions are commonly prepared from the heptahydrate form (MgSO₄·7H₂O, MW = 246.47 g/mol) because the anhydrous form is very hygroscopic and difficult to weigh accurately. A 1 M stock solution using MgSO₄·7H₂O is a standard reagent for many biochemical and cell culture applications.
Objective
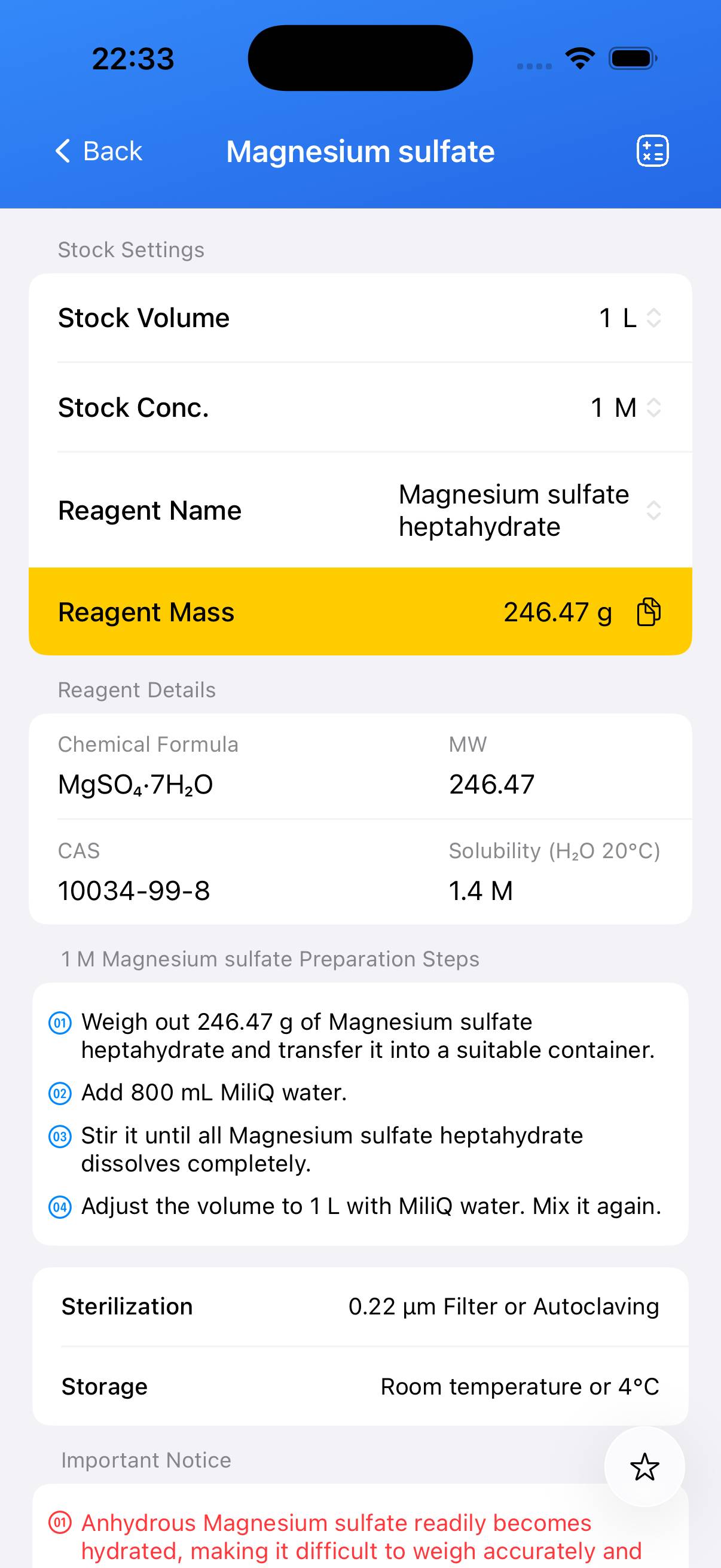
Preparation of a 1 M magnesium sulfate stock solution (e.g., 100 mL).
Composition
- Magnesium sulfate (MgSO₄·7H₂O) aqueous solution at defined molarity
Required Materials
- Magnesium sulfate heptahydrate (MgSO₄·7H₂O)
- Deionized or distilled water
- Analytical balance
- Beaker or flask
- Graduated cylinder or volumetric flask
- Stirring rod or magnetic stirrer
- Personal protective equipment (gloves, goggles, lab coat)
Procedure
- Step 1
To prepare 100 mL of 1 M MgSO₄ stock solution, accurately weigh **24.65 g** of magnesium sulfate heptahydrate (MgSO₄·7H₂O). - Step 2
Add the weighed salt to ~80 mL of deionized water in a beaker. Stir or swirl until completely dissolved. - Step 3
Transfer the solution to a volumetric flask and bring the final volume up to **100 mL** with deionized water. Mix thoroughly. - Step 4 (Optional)
For sterile applications, sterilize by autoclaving (121°C, 15–20 min) or filter through a **0.22 µm** membrane into a sterile container.
Note: Do not dissolve all solute directly in the final volume; large amounts of solute increase solution volume. Autoclaving is optional for non‑sterile use.
Available Reagents
| Name | Molecular Formula | MW | CAS |
|---|---|---|---|
| Magnesium sulfate heptahydrate | MgSO₄·7H₂O | 246.47 | 10034‑99‑8 |
| Magnesium sulfate monohydrate | MgSO₄·H₂O | 138.38 | 14168‑73‑7 |
| Anhydrous magnesium sulfate | MgSO₄ | 120.37 | 7487‑88‑9 |