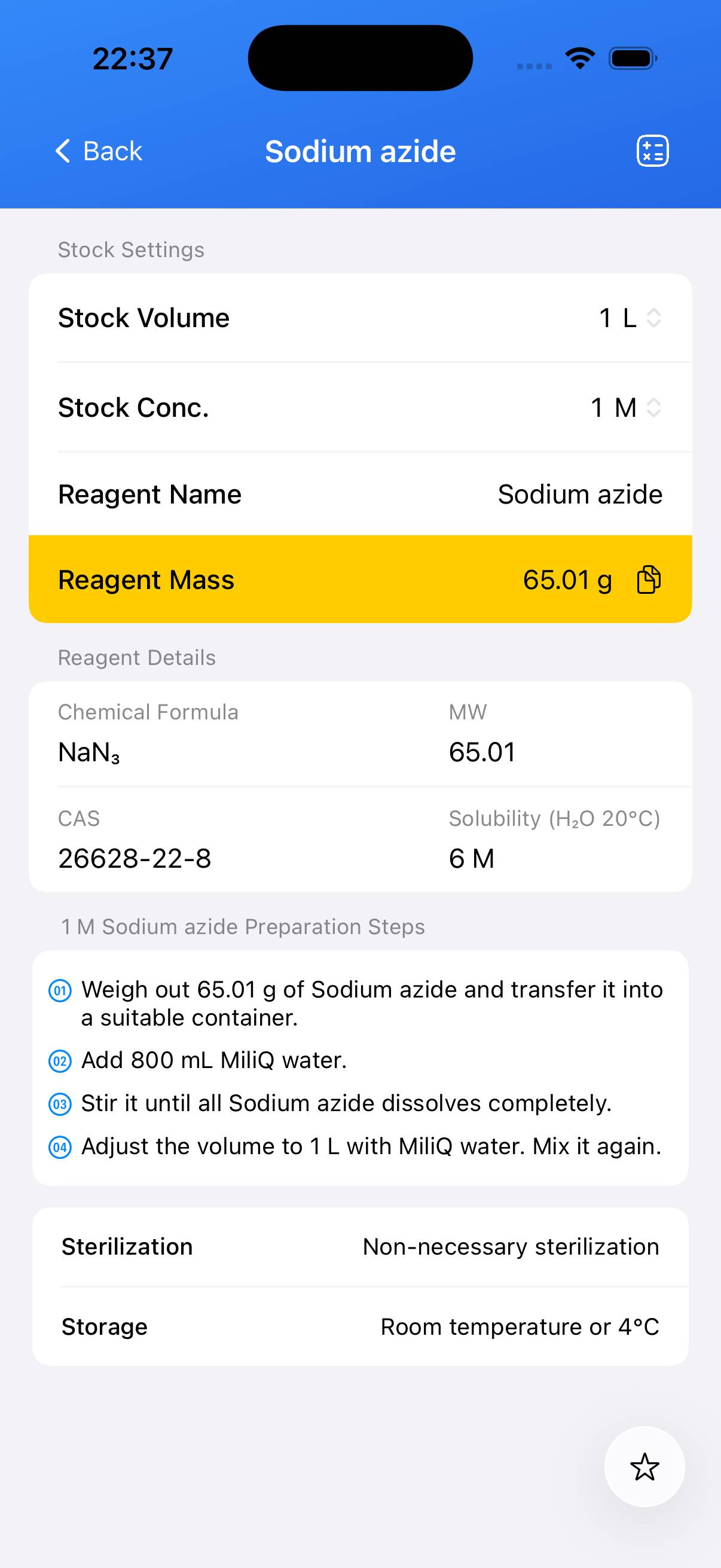
Sodium azide Stock Solution
This guide describes the preparation of Sodium azide at a defined molarity for labo xxxrStock Solutionry use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – 10% Sodium Azide (NaN₃) Solution
Sodium azide is supplied commercially as a white crystalline powder and is commonly added to reagent solutions to prevent microbial growth. It is also a potent inhibitor of some enzyme activities. It is highly toxic and reacts with metals and acids to form hazardous compounds; therefore, careful handling and safety precautions are essential.
Objective
Preparation of a 10% (w/v) sodium azide aqueous stock solution for preservative use.
Composition
- 10% (w/v) sodium azide (NaN₃) in deionized/Milli‑Q water
Required Materials
- Sodium azide (NaN₃) solid
- Deionized or Milli‑Q water
- 15 mL disposable graduated tube or appropriate container
- Electronic balance
- Non‑metallic spatula (to avoid formation of explosive metal azides)
- Tube rotator or manual mixing
- Personal protective equipment (lab coat, nitrile gloves, chemical goggles)
Procedure
- Step 1
Wear appropriate PPE and work in a chemical hood. Accurately weigh **1 g** of sodium azide powder into a 15 mL disposable graduated tube. Handle the solid with care and avoid direct contact; sodium azide is **highly toxic**. - Step 2
Add **8 mL** deionized or Milli‑Q water to the tube. Close the tube and invert several times or use a tube rotator to mix until fully dissolved. Sodium azide dissolves readily in water. - Step 3
Adjust the final volume to **10 mL** with additional water to obtain a **10% (w/v)** solution (1 g NaN₃ in 10 mL water).
Storage: Store the 10% sodium azide solution at room temperature in a clearly labeled, tightly closed chemical bottle away from acids and metals. Include hazard information on the label.
Safety Notice
Sodium azide is acutely toxic and hazardous by inhalation, ingestion, or skin contact. Avoid contact with metals as this can produce explosive azides and avoid acidic conditions to prevent formation of toxic hydrazoic acid. Follow institutional hazardous waste disposal procedures for sodium azide solutions.
Available Reagents
| Name | Molecular Formula | MW | CAS |
|---|---|---|---|
| Sodium azide | NaN₃ | 65.01 | 26628‑22‑8 |