Sodium bicarbonate Stock Solution
This guide describes the preparation of Sodium bicarbonate Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Sodium Bicarbonate (NaHCO₃) Solution
Sodium bicarbonate (NaHCO₃), also known as baking soda, is a white crystalline powder that is highly soluble in water and mildly alkaline. A standard 1 M sodium bicarbonate solution has uses in buffering, titration analysis, and pH control. The molecular weight of NaHCO₃ is approximately 84.01 g/mol, and the solution at 25 °C has a pH around 8.3 when at 1 M concentration.
Objective
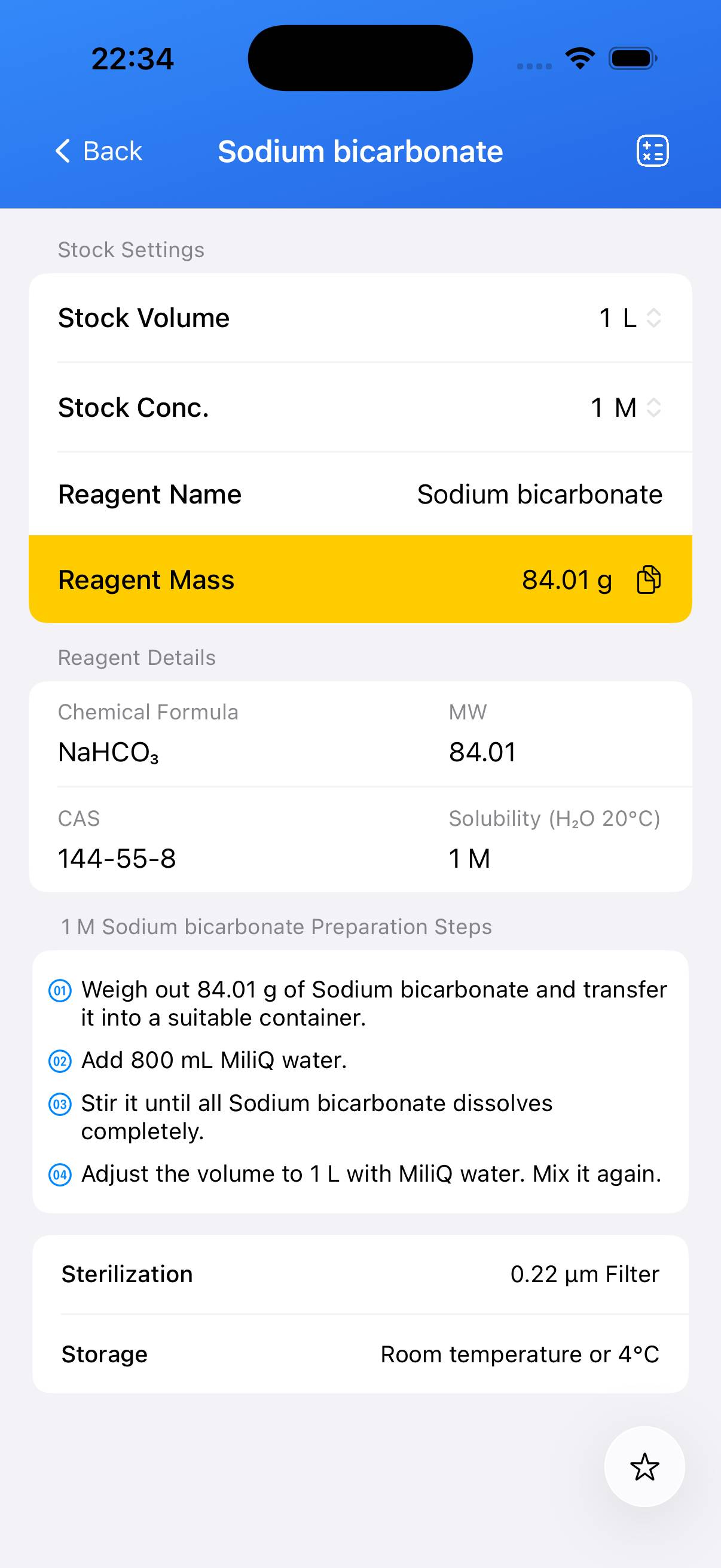
Preparation of a 1 M sodium bicarbonate stock solution.
Composition
- Sodium bicarbonate (NaHCO₃) aqueous solution of defined molarity
Required Materials
- Sodium bicarbonate solid (NaHCO₃)
- Deionized or distilled water
- Analytical balance
- Beaker or flask
- Volumetric flask or graduated cylinder
- Stirring rod or magnetic stirrer
- Personal protective equipment (gloves, goggles, lab coat)
Procedure
- Step 1
Decide the target concentration. For a 1 M solution: accurately weigh **84.01 g** of sodium bicarbonate. - Step 2
Add the weighed sodium bicarbonate solid to a beaker containing approximately **80 % of the final desired volume** of deionized water (e.g., ~800 mL if preparing 1 L). Stir until completely dissolved. - Step 3
Transfer the solution to a volumetric flask and rinse the beaker with small amounts of water, adding rinses to the flask. Add deionized water to reach the final target volume (e.g., 1 L). Mix thoroughly. - Step 4 (Optional)
If a sterile solution is required for sensitive applications, filter through an appropriate membrane filter (e.g., 0.22 µm) into a sterile container. Sterilization by heat is generally not necessary for most applications but follow your protocol requirements.
Note: A 1 M NaHCO₃ solution typically has a pH around 8.3 at room temperature. Label the container with concentration and preparation date. Store the stock solution in a sealed container at room temperature or as required.
Available Reagents
| Name | Molecular Formula | MW | CAS |
|---|---|---|---|
| Sodium bicarbonate | NaHCO₃ | 84.01 | 144‑55‑8 |