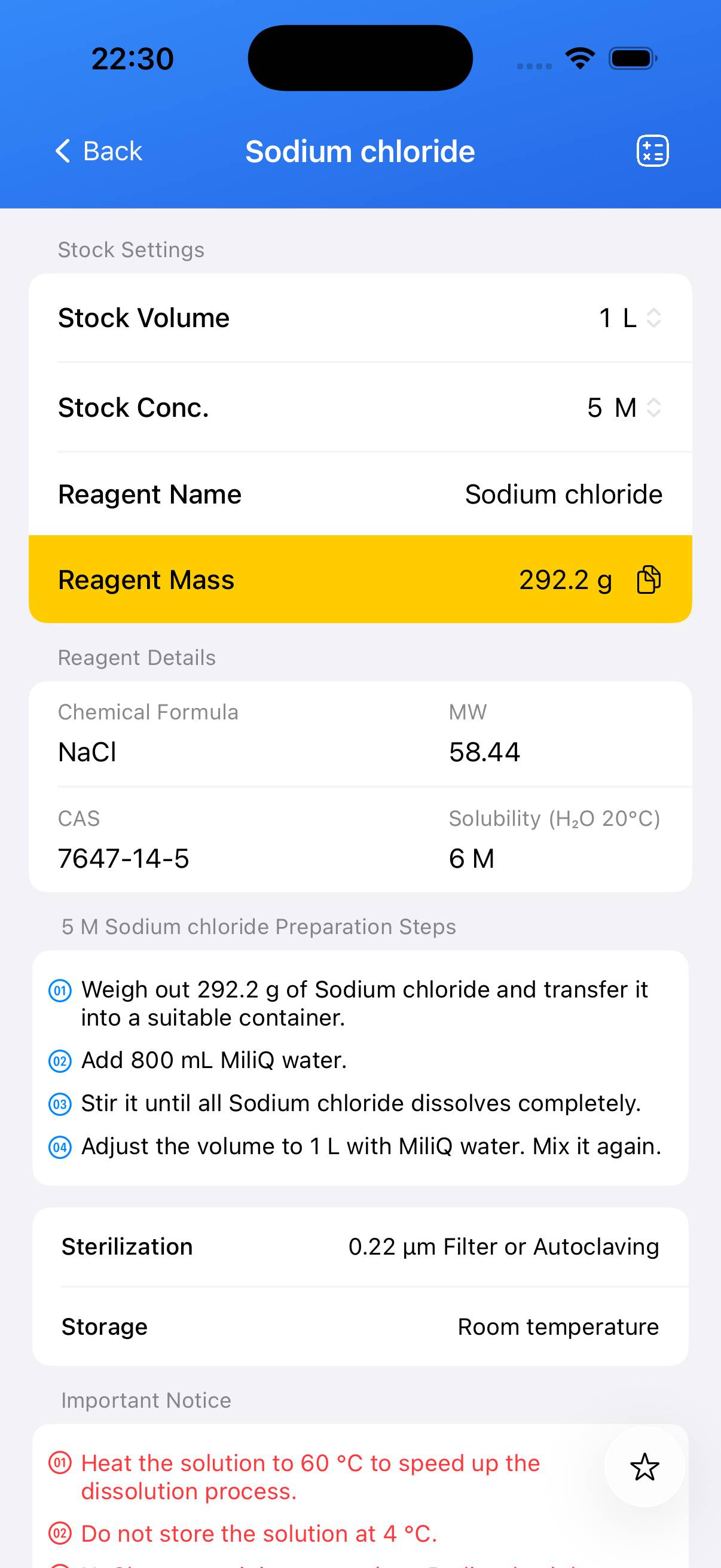
Sodium chloride Stock Solution
This guide describes the preparation of Sodium chloride Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Sodium Chloride (NaCl)
Sodium chloride (NaCl) is a simple inorganic salt widely used in biological, biochemical, and chemical laboratories. It functions as an ionic strength adjuster, a key component in buffer formulations, saline solutions, and cell culture media. The molar mass of sodium chloride is 58.44 g/mol, and this value is used to calculate the mass required for a desired molar concentration.
Chemical Information
| Name | Molecular Formula | Molecular Weight | CAS |
|---|---|---|---|
| Sodium chloride | NaCl | 58.44 g/mol | 7647-14-5 |
Preparation of a 1 M NaCl Solution
A typical procedure for preparing 1 L of a 1 M sodium chloride stock solution is as follows:
- Calculate the required mass for 1 mol: 58.44 g of NaCl for 1 L of a 1 M solution.
- Weigh out 58.44 g of sodium chloride on an analytical balance.
- Place the NaCl into a beaker or volumetric flask and add about 700–800 mL of deionized or distilled water.
- Stir until the salt is completely dissolved.
- Transfer the solution to a 1 L volumetric flask (if not already in one) and add deionized water to bring the final volume to exactly 1000 mL.
- Cap and mix the solution thoroughly; the 1 M NaCl stock is now ready for use.
Notes and Precautions
- Ensure accurate weighing of NaCl to achieve correct molarity.
- Do not add all the solid directly to the full final volume of water; add to part of the water first and then dilute to the final volume.
- Wear appropriate personal protective equipment (lab coat, gloves, goggles) during preparation.
Storage and Usage
- The prepared 1 M NaCl solution can be stored at room temperature or refrigerated if required.
- Common applications include buffer preparation (e.g., PBS), saline solutions, and use as ionic strength modifier in biochemical assays.