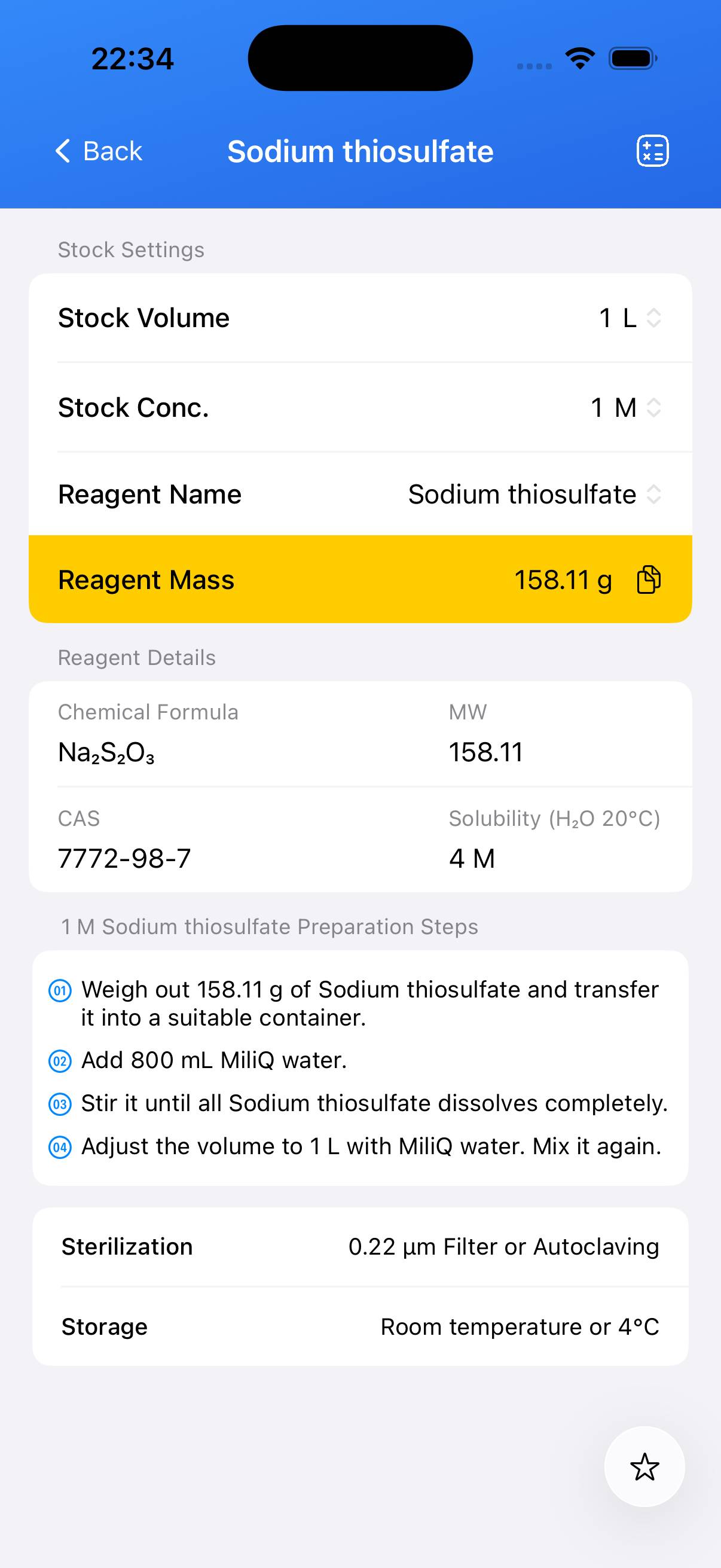
Sodium thiosulfate Stock Solution
This guide describes the preparation of Sodium thiosulfate Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Sodium Thiosulfate (Na₂S₂O₃) Solution
Sodium thiosulfate (Na₂S₂O₃) and its hydrated form sodium thiosulfate pentahydrate (Na₂S₂O₃·5H₂O) are commonly used reagents in analytical chemistry and titration work. Standard stock solutions (e.g., 0.1 M) are frequently prepared for quantitative analyses or as intermediate solutions in redox titrations.
Objective
Preparation of a defined‑concentration stock solution of sodium thiosulfate (e.g., 0.1 M).
Composition
- Sodium thiosulfate (Na₂S₂O₃ or Na₂S₂O₃·5H₂O) aqueous solution at defined molarity
Required Materials
- Sodium thiosulfate (anhydrous or pentahydrate)
- Deionized or distilled water
- Analytical balance
- Beaker or flask
- Volumetric flask or graduated cylinder
- Stirring rod or magnetic stirrer
- Personal protective equipment (gloves, goggles, lab coat)
Procedure
- Step 1
Decide the target concentration. For a 0.1 M Na₂S₂O₃ solution: accurately weigh the required mass of sodium thiosulfate pentahydrate or calculate for the anhydrous form. Using pentahydrate, **≈25 g** of Na₂S₂O₃·5H₂O dissolved to **1 L** gives ~0.1 M solution. Anhydrous Na₂S₂O₃ requires a lower mass (~16 g for 1 L). - Step 2
Place the weighed solid into a beaker containing approximately **~80% of the final desired volume** of deionized water. Stir until fully dissolved; gentle warming can aid dissolution if necessary. - Step 3
Transfer the solution to a volumetric flask. Rinse the beaker with small amounts of water and add rinses to the flask. Add deionized water to bring the total volume up to the final target (e.g., 1 L). Mix thoroughly. - Step 4 (Optional)
Prepare the solution under low light or label the container with concentration, preparation date, and storage conditions. For long‑term use, store in a sealed container away from heat and direct light.
Note: Sodium thiosulfate solutions are generally stable at room temperature when stored properly. If used for titrimetric standards, follow appropriate calibration or standardization procedures before analytical use.
Available Reagents
| Name | Molecular Formula | MW | CAS |
|---|---|---|---|
| Sodium thiosulfate | Na₂S₂O₃ | 158.11 | 7772‑98‑7 |
| Sodium thiosulfate pentahydrate | Na₂S₂O₃·5H₂O | 246.18 | 10102‑17‑7 |