Preparation EGTA Stock Solution
This guide describes the preparation of EGTA Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – EGTA
EGTA (Ethylene Glycol Tetraacetic Acid) is a highly selective calcium ion chelator used in biochemical and cell biology experiments to control free Ca2+ concentrations. EGTA binds calcium ions with much higher affinity compared to magnesium, making it useful for buffering and chelation applications where calcium-specific sequestration is needed.
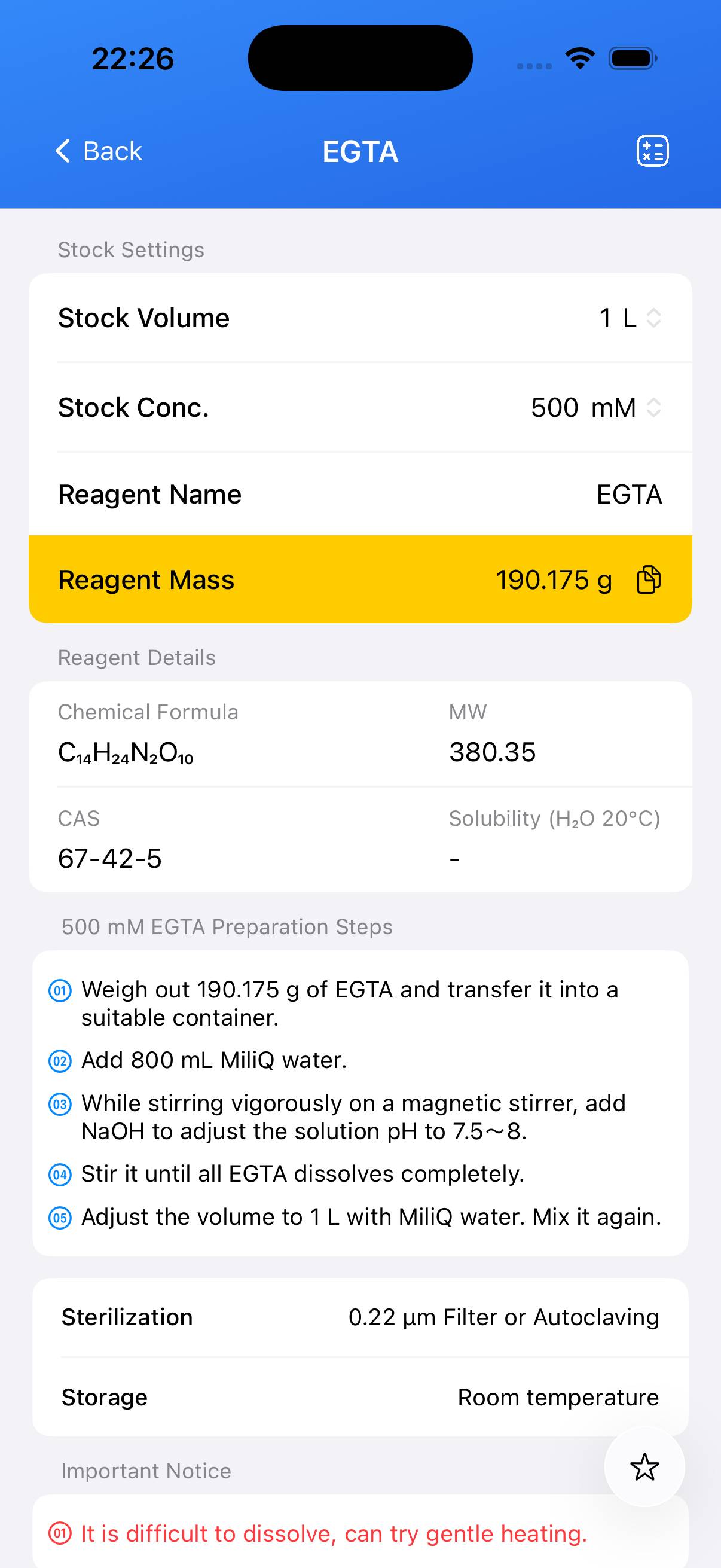
Chemical Properties
- CAS Number: 67-42-5
- Molecular Formula: C14H24N2O10
- Molecular Weight: 380.35 g/mol
Solubility and Handling
EGTA is only slightly soluble in water and typically requires alkaline conditions (for example, addition of NaOH) to fully dissolve. In practice, stock solutions are first made in water adjusted to basic pH to ensure dissolution, or in small volumes of concentrated NaOH followed by dilution.
- Soluble in alkaline aqueous solutions; precipitation can occur if the pH drops.
- Limited solubility in water at neutral pH; warming and sonication may aid solubilization.
Stock Solution Preparation
Below are example volumes to prepare EGTA stock solutions at common molarities. Adjust the amounts based on the exact batch molecular weight if necessary.
5 mM: 0.53 mL per 1 mg
10 mM: 0.26 mL per 1 mg
After dissolving, adjust pH if required, bring to final volume with buffer or water, and sterile-filter the solution if needed.
Storage and Usage
- Stock solutions can be aliquoted and stored at 2-8°C for several months.
- Avoid repeated freeze–thaw cycles; prepare working dilutions just prior to use.
- Use sterile techniques and filtration to avoid contamination for cell culture applications.
Applications
EGTA is commonly used to chelate calcium in biochemical assays, prepare calcium-controlled buffers, and modulate intracellular or extracellular Ca2+ levels in cell biology studies. Its high selectivity for calcium over magnesium makes it especially useful where differential ion chelation is required.