Preparation Tris-Cl Stock Solution
This guide describes the preparation of Tris-Cl Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Tris Buffer (1 M, pH 7.2)
Tris (Tris(hydroxymethyl)aminomethane), also known as Trizma or THAM, is a widely used biological buffer in molecular biology and biochemistry. It has a pKa of ~8.08 at 25 °C and an effective buffering range between ~7.2 and 9.0, making it suitable for physiological pH applications. The solution’s pH is temperature dependent and should be adjusted at the temperature of intended use.
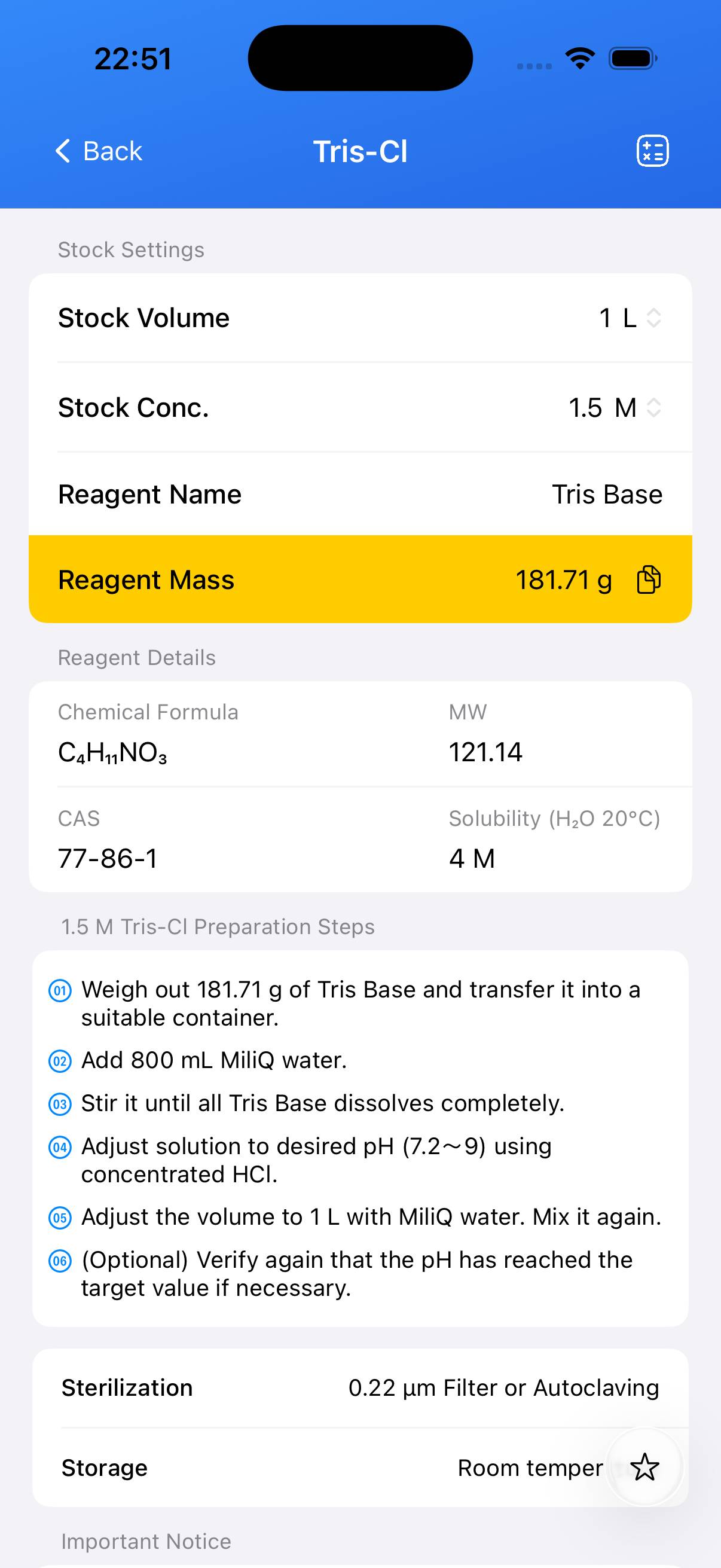
Chemical Information
| Name | Molecular Formula | Molecular Weight | CAS |
|---|---|---|---|
| Tris Base | C₄H₁₁NO₃ | 121.14 g/mol | 77-86-1 |
Applications
- General biological buffering between pH 7.2–9.0
- Preparation of TAE, TBE, and TE buffers
Solubility and Handling
- Tris base is a white powder that dissolves readily in water.
- Because pH changes with temperature, adjust the pH at the temperature at which the buffer will be used.
Preparation of 1 M Tris.Cl (pH 7.2)
- Weigh 121.14 g Tris base and transfer into a 1 L beaker or flask.
- Add ~800 mL deionized water and dissolve with stirring.
- Allow the solution to cool to room temperature if warmed. Do not adjust pH while hot.
- Slowly add concentrated HCl (≈ 45 mL) while monitoring the pH until it reaches 7.2.
- Transfer to a 1 L graduated cylinder and adjust the final volume to 1000 mL with deionized water.
- Optional: Autoclave for 20 min at 15 psi if sterile buffer is required.
Storage and Notes
- 1 M Tris.Cl pH 7.2 is clear and colorless; discard if yellowish.
- Store at room temperature for several weeks.
Preview