Preparation PIPES Stock Solution
This guide describes the preparation of PIPES Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – PIPES
PIPES (Piperazine-N,N′-bis(2-ethanesulfonic acid) and its sodium salt forms are zwitterionic Good’s buffers commonly used in biochemical and cell biology experiments. PIPES operates effectively in the pH range of approximately 6.1–7.5 and has minimal interaction with divalent metal ions, making it suitable for buffering applications where ion neutrality is important.
Chemical Information
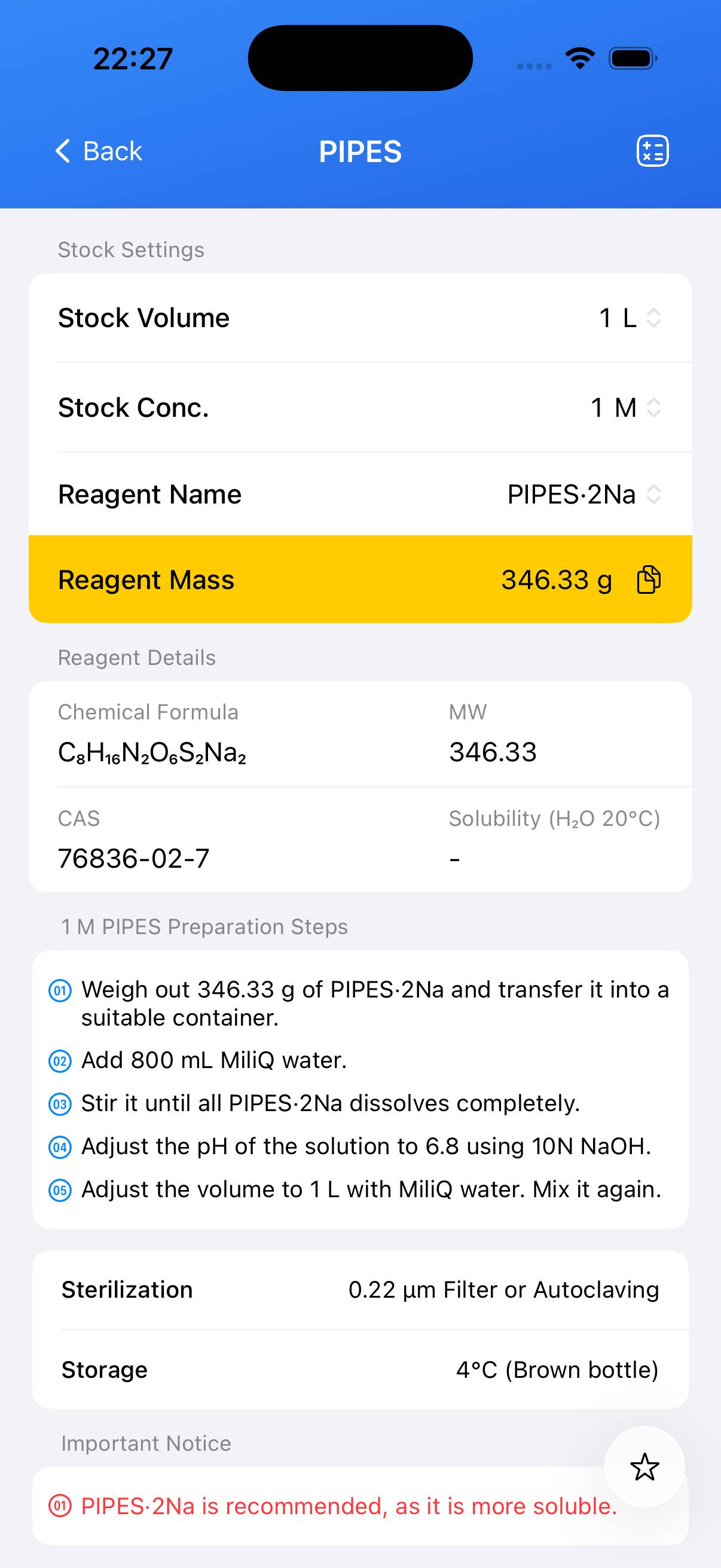
- CAS Number: 5625-37-6
- Molecular Formula: C₈H₁₈N₂O₆S₂
- Common Salt Forms: PIPES·2Na, PIPES·Na, PIPES·1.5Na, PIPES (Free Acid)
- Molecular Weights: Various depending on salt form (e.g., free acid 302.38 g/mol, disodium 346.33 g/mol). See reagent list for details.
Buffer Properties and Uses
- PIPES is a zwitterionic biological buffer with useful pH buffering capacity near neutral conditions.
- It does not significantly chelate metal ions, useful for experiments containing divalent cations.
- Used in protein purification, chromatography, cell culture media, and fixation solutions for microscopy.
Solubility and Handling
- Free acid form has limited solubility in water and typically requires pH adjustment with NaOH to dissolve effectively.
- Sodium salt forms are more soluble and convenient for direct buffer preparation.
- PIPES buffer solutions can be sterilized by filtration if required for cell culture.
Stock Solution Preparation
To prepare a PIPES buffer stock at a specific molarity:
• Add 302.37 g of PIPES free acid to ~800 mL deionized water.
• Slowly add NaOH (e.g., 5–10 N) while stirring to dissolve and adjust pH to target (typically ~6.8).
• Once dissolved and pH adjusted, bring to final volume of 1 L with water.
Alternatively, mix equimolar amounts of monosodium and disodium PIPES salts and titrate to the desired pH.
Storage and Use
- Store solid PIPES reagents dry at room temperature; buffer solutions can be stored refrigerated.
- Filter sterilize buffer solutions when required for sensitive biological applications.
Available Reagents
| Name | Molecular Formula | Molecular Weight (g/mol) | CAS |
|---|---|---|---|
| PIPES·2Na | C₈H₁₆N₂O₆S₂Na₂ | 346.33 | 76836-02-7 |
| PIPES·Na | C₈H₁₇N₂O₆S₂Na | 324.35 | 10010-67-0 |
| PIPES·1.5Na | C₈H₁₆.₅N₂O₆S₂Na₁.₅ | 335.34 | 100037-69-2 |
| PIPES (Free Acid) | C₈H₁₈N₂O₆S₂ | 302.38 | 5625-37-6 |